You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Peri-implantitis has been defined as an inflammatory process around osseointegrated implants in function affecting the mucosa and resulting in loss of supporting bone around the implant.1 Peri-implantitis is differentiated from peri-implant mucositis, which is a term used to describe a reversible inflammatory reaction confined to the mucosa around the implant. Both of these peri-implant diseases have similar clinical signs and symptoms, including bleeding following gentle probing, increased probing depths, and/or suppuration.2-4 A diagnosis of peri-implantitis is made when the above-mentioned signs and symptoms are present together with evidence of radiographic bone loss around the implant. In cases of peri-implantitis, this bone loss exceeds that which Albrektsson described as being physiologically acceptable to consider an implant successful (no more than 0.2 mm per year following 1-year loading of the implant).5 Although the prevalence of peri-implantitis has been reported in several studies to vary from approximately 11% to 47%, even the low end of this range denotes a significant problem/challenge for the dental team.4,6
The etiology of peri-implantitis has been associated with plaque accumulation combined with a susceptible host response.7,8 Recently, triggering factors described for peri-implantitis have included “lesions of peri-implant attachment, presence of aggressive bacteria, excessive mechanical stress, and corrosion.”9 If validated, this may present a multifactorial synergy for the etiology and progression of peri-implantitis. Nevertheless, for any treatment to be effective, the primary etiologic agent—bacterial biofilm—must be both eliminated from the lesion and the implant surface and prevented from becoming re-established.
A number of systematic reviews regarding the treatment of peri-implantitis have concluded that non-surgical therapy and surgical debridement—with or without the use of local or systemic antibiotics—failed to provide predictable long-term successful outcomes.10-15 Recently, a case series documented a successful regenerative treatment protocol for peri-implantitis-affected implants, which were followed for 3 to 7.5 years post-treatment.16 The success of these cases relied on a team approach between the surgeon and the restorative dentist. This collaboration requires early diagnosis, knowledge of both the etiology and risk factors associated with peri-implantitis to enable their elimination, a meticulous surgical protocol, and stringent professional and patient-performed maintenance. Each of these areas will be elaborated upon as they relate to successful outcomes when using this specific regenerative approach.
Diagnosis
As noted in several human studies, early diagnosis and treatment is recommended for the most predictable results of therapy.17,18 Since the primary care dentist and his/her hygienist usually examine and treat patients prior to any referral, they are in the best position to initially detect peri-implantitis. This is accomplished by clinical examination and noting inflamed mucosa, bleeding on light probing and/or suppuration, and probing depths (PD) > 3 mm, which diagnose the presence of peri-implant pathology. As with natural teeth, probing should be performed with a rounded UNC periodontal probe around six aspects of the implant. Bleeding on probing (BoP) should be assessed and can be reported using a dichotomous index by waiting a period of 15 seconds after light probing and recording a positive (or negative) result if bleeding is noted.19 If the above clinical signs are present, a periapical radiograph should be exposed and bone levels should be assessed and compared to previous radiographs. Using a proposed peri-implantitis classification, which measures bone loss relative to known implant length, will then allow a comparison of bone levels even in cases where radiographs are not standardized. Loss of supporting bone, together with signs of inflammation, denote peri-implantitis, and a diagnosis of early, moderate, or severe disease can then be made.20
Implant mobility is not a useful diagnostic parameter. If an implant is mobile, it should be considered failed and removed. Conversely, implants may have advanced peri-implantitis with bone loss from 50% to 90% of implant length and still exhibit no mobility when examined clinically (Figure 1).
Pathogenesis
Peri-implantitis has been reported to be a site-specific infection.21 Therefore, probing of each implant should be performed at every recall visit, measuring and recording any increase in probing depth compared to previous examinations. Bone loss associated with peri-implantitis has been demonstrated to be non-linear.17 The clinician must be aware that any increase in PD may be indicative of possible ongoing progression of peri-implantitis. One study that compared peri-implant disease to chronic and aggressive periodontitis concluded that although the etiology, pathogenesis, and risk assessment of these diseases “are not fundamentally different,” some “differences in the host response to these two infections may explain the occasional rapid progression of peri-implantitis lesions.” The authors strongly recommended that once diagnosed, “peri-implantitis should be treated without delay.”22 Moreover, Salvi et al reported that “peri-implant soft tissues developed a stronger inflammatory response to experimental plaque accumulation when compared with those of their gingival counterparts.”23 Lastly, Berglundh et al, using human biopsy material, reported that the apical extension of the inflammatory cell infiltrate “was more pronounced in peri-implantitis than in periodontitis and was in most cases located apical of the pocket epithelium.”24 The above-mentioned findings should alert the clinician to the importance of performing a thorough assessment of all peri-implant conditions at every recall maintenance visit, and of the urgency for treatment or referral when indicated.
Risk Factors
The Consensus Report of the Sixth European Workshop on Periodontology listed the following risk indicators associated with peri-implant diseases: poor oral hygiene, a history of periodontitis, and cigarette smoking. Other possible risk factors noted were diabetes with poor metabolic control, alcohol consumption, genetic traits, and the implant surface.11 The latter was discussed in an animal study, which concluded that once experimental peri-implantitis with bone loss was established and left untreated, none of the four implant surfaces studied (TiOblast™ [DENTSPLY Implants, www.dentsplyimplants.com], Straumann SLA® Implant Surface [Straumann, www.straumann.com], TiUnite™ [Nobel Biocare, www.nobelbiocare.com], or machined) was resistant to the spontaneous progression of bone loss. The entire dental team—primary care dentist, hygienist, and periodontist—must be knowledgeable of and vigilant towards these risk factors when maintaining the oral health of patients with implant restorations. The team must also inform the patient who has any of these risk indicators that they are at higher risk of developing peri-implantitis around their implant restorations.
One additional risk factor not mentioned in the aforementioned consensus report, but that is quite commonly seen in association with peri-implantitis, is retained cement on the surface of implants following cementation of the restorations. Wilson, in a study using an endoscope, found excess dental cement associated with signs of peri-implant disease in 81% of the implants evaluated.25 Moreover, after removal of this cement—again using an endoscope to aid in evaluating cement-free surfaces—signs of disease were absent in 74% of the test implants. However, these results also imply that 26% of the implants still had signs of the disease even after thorough cement removal. Two recently published studies have discussed factors that could influence the effectiveness of excess cement removal. The first concluded that some types of cements commonly used for cementation of implant-supported prostheses may not be detectable following radiographic examination because of their poor radiolucency.26 The second study concluded that the amount of residual cement increased as the restorative margin was located more apically despite efforts to remove it.27 The importance of careful cementation by the restorative dentist is emphasized by these studies. Furthermore, the factors discussed in these articles underscore the importance of ensuring that the dental team thoroughly examine the subgingival implant and abutment surfaces visually for residual cement or to consider the use of a mini-access flap to gain proper visualization.
Elimination of Etiology
The importance of establishing periodontal health prior to both implant placement and the treatment of peri-implantitis has been discussed in numerous studies.22,28,29 In addition, properly contoured restorations on implants are essential in allowing a patient to maintain good plaque control following any surgical intervention. This may entail removal of an ill-fitting or poorly contoured crown and creating a well-fitting provisional prior to surgery (Figure 2 and Figure 3). In a study of 23 subjects who presented for treatment of peri-implantitis, it was reported that 74% of their implants diagnosed with peri-implantitis were associated with no accessibility/capability for proper oral hygiene. The authors emphasized the importance of proper prosthetic constructions that allow accessibility for oral hygiene around implants.30 Patients should also be taught proper homecare methods prior to surgery, to minimize plaque accumulation during and following treatment to ensure optimum healing of the peri-implant tissues.
Surgical Protocol
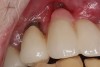
A recently published prospective case series described the technique, materials, and rationale for a protocol to successfully treat peri-implantitis with a regenerative approach.16 The results reported in the study were unique in that in addition to an average bone fill of more than 3 mm of the defect, soft-tissue levels post-surgery showed an average of more than 1 mm of coronal coverage of the implant surfaces (Figure 4 and Figure 5).
To briefly review, the seven essential steps in this technique are as follows:
1. There should be adequate full-thickness flap reflection to allow access to the contaminated implant surface and osseous defect. Vertical and periosteal incisions are used to aid in obtaining access and providing a non-tension closure after suturing.
2. Surface decontamination using special graphite and titanium-tipped curettes is followed by a six-step protocol using an air-abrasive device, first with fine powder, and finally with saline alone for 60 to 90 seconds. Between these sprays, tetracycline 50 mg/ml (or minocycline 50 mg/ml) and 0.12% chlorhexidine gluconate are applied to the implant surface with cotton pellets or a dedicated sterile brush.
3. Enamel matrix derivative is then applied to the decontaminated implant surface and surrounding bone.
4. The defect is filled and the exposed implant surfaces are covered with either anorganic bovine bone or mineralized bone allograft or a combination of the two, which is hydrated with platelet-derived growth factor at least 5 minutes prior to graft placement.
5. Where there is an adequate band of keratinized tissue (KT) ≥ 2 mm preoperatively, the graft is covered with two resorbable membranes—one on the facial and one on the lingual—and contoured to cover the interproximal bone. In cases where the amount of presurgical KT is inadequate, a subepithelial connective tissue (sect) graft, obtained from the palate, is used in place of the resorbable membrane. When using a sect graft, it too must cover all aspects of the implant surface and grafted bone defect.
6. The flap is coronally advanced and secured with absorbable or nonresorbable monofilament sutures, with nontension closure, to completely cover the bone graft and membrane or sect graft.
7. During and following healing, daily proper homecare and professional maintenance at a minimum interval of every 3 months is essential.
The following case report depicts the regenerative procedure and outcomes, and demonstrates the importance of regular professional care in maintenance of a successful result (Figure 6 through Figure 16).
Case Report
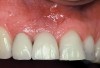
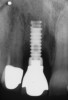
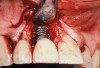
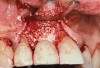
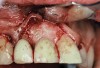
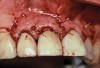
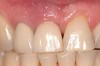
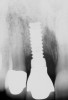
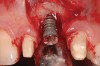
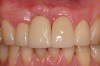
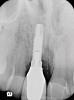
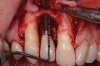
A 46-year-old man with an unremarkable medical history was referred for evaluation and treatment of the maxillary right central incisor implant. The implant, which had been placed 6 years prior, had never caused any problems until recently, when the patient noted both swelling and soreness at the site. Following 10 days of amoxicillin 500 mg tid, purulence was still present upon palpation of the tissue, and the patient was referred for consultation (Figure 6). Probing attachment loss of 10 mm on the distal aspect and 5 mm on the mesial were measured around the implant. Bone loss was advanced on the periapical radiograph at both the mesial and distal aspects of the hydroxyapatite-coated implant (Figure 7). Full-thickness facial and lingual flaps were reflected to access the implant and surrounding bone, with care taken to preserve the papilla. The defects around the implant on the mesial and distal were 1-wall, and bone loss was present on the direct facial and palatal aspects as well (Figure 8). The surface of the implant was decontaminated, followed by application of enamel matrix derivative. The lesion was filled with freeze-dried bone allograft (Figure 9). The graft-biologic was covered by a flowable polymer barrier (Figure 10). The flaps were coronally advanced and secured with monofilament sutures (Figure 11). The patient was prescribed amoxicillin with clavulanic acid and an oral rinse of 0.12% chlorhexidine during the post-operative period. The healing progressed uneventfully, achieving both a favorable soft-tissue profile and substantial bone fill, as determined radiographically. These results were stable for 7 years (Figure 12 and Figure 13).
The patient returned regularly for recall visits for 7 years, but then did not return for follow-up or maintenance for 2 years. He then returned for evaluation and retreatment of the implant. However, retreatment was not possible as the bone loss and lesion morphology were unfavorable for attempting a regenerative approach (Figure 14). The implant was removed atraumatically, and the site was grafted with a composite graft of mineralized and demineralized freeze-dried bone allograft and covered with an absorbable porcine collagen barrier. The flap was advanced to enable primary closure of the site and it was allowed to heal for 6 months. Re-entering the site, the bone fill achieved by the regenerative efforts enabled a second implant to be placed in a favorable prosthetic position (Figure 15 and Figure 16).
Maintenance and Monitoring
As seen in this case, the long-term success of this regenerative surgical approach depends on the ability of the patient and the collaborative treatment team to maintain excellent plaque control. An obvious successful outcome using this regenerative approach was later lost when the patient did not present for regular follow-up, maintenance, and monitoring. A systematic review of the literature concluded that few studies were available to evaluate the long-term effect of supportive programs and that there was no evidence to suggest frequency of recall intervals.31 However, a 3-year prospective case control study following surgical treatment of peri-implant lesions reported stable bone fill during the observation period when patients were held to a strict maintenance program with professional cleaning visits every 3 months.32
Another retrospective study of 1626 implants with follow-up ranging from 1 to 114 months (average 30.82 months) concluded that attendance in a regular supportive periodontal program was found to be strongly related to implant survival.33 Regular maintenance and monitoring following surgery aimed at regeneration of soft and hard tissue as well as reosseointegration should begin approximately 3 months post-surgery, following the early phase of post-surgical care (weekly for 4 to 6 weeks, then every 2 weeks for the next 6 to 8 weeks).
Although a number of articles have been written on the topic of implant maintenance and peri-implant homecare, some additional procedures are recommended based on current research.34 Several studies have discussed the use of rubber cups and flour of pumice and water being effective while causing little damage to the implant surface.35 In addition, the effectiveness of air-polishing devices has also been documented, with a recent study concluding that for rough implant surfaces, non-metal instruments and air abrasives were the instruments of choice.36 The author advocates these two methods of maintaining implants at recall, as well as the use of special curettes previously mentioned in this article.
Conclusion
Prevention of peri-implantitis and maintenance of implants successfully treated with a regenerative approach is the collaborative responsibility of the primary care dentist, hygienist, and periodontist. Probing depth, the presence of BoP and/or suppuration, and radiographs should be assessed regularly to diagnose peri-implant disease. Early diagnosis and treatment has been shown to result in the best outcomes when attempting to save peri-implantitis-affected implants. The effectiveness of the regenerative approach to treat peri-implantitis as described herein depends on this collaborative treatment.
ACKNOWLEDGEMENT
Case report information was supplied by Paul S. Rosen, DMD, MS of Yardley, Pennsylvania.
REFERENCES
1. Albrektsson T, Isidor F. Consensus report of session IV. In: Lang NP, Karring T, eds. Proceedings of the First European Workshop on Periodontology. London: Quintessence Publishing; 1994:365-369.
2. Mombelli A. Criteria for success. Monitoring. In: Lang NP, Karring T, eds. Proceedings of the First European Workshop on Periodontology. London: Quintessence Publishing;. 1994:317-325.
3. Mombelli A. Prevention and therapy of peri-implant infections. In: Lang NP, Karring T, Lindhe J, eds. Proceedings of the 3rd European Workshop on Periodontology. Berlin: Quintessenz Verlag; 1999:281-303.
4. Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008;35(8 Suppl):286-291.
5. Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1(1):11-25.
6. Koldsland OC, Scheie AA, Aass AM. Prevalence of peri-implantitis related to severity of the disease with different degrees of bone loss. J Periodontol. 2010;81(2):231-238.
7. Mombelli A, Lang NP. The diagnosis and treatment of peri-implantitis. Periodontol 2000. 1998;17:63-76.
8. Lang NP, Berglundh T; Working Group 4 of Seventh European Workshop on Periodontology. Peri-implant diseases: where are we now?—Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011;38(Suppl 11):176-181.
9. Mouhyi J, Dohan Ehrenfest DM, Albrektsson T. The peri-implantitis: implant surfaces, microstructure, and physiochemical aspects. Clin Implant Dent Relat Res. 2012;14(2):170-183.
10. Roos-Jansåker AM, Renvert S, Egelberg J. Treatment of peri-implant infections: A literature review. J Clin Periodontol. 2003;30(6):467-485.
11. Lindhe J, Meyle J; Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35(8 Suppl):282-285.
12. Claffey N, Clark E, Poluzois I, Renvert S. Surgical treatment of peri-implantitis. J Clin Periodontol. 2008;35(8 Suppl):316-332.
13. Kotsovilis S, Karoussis IK, Trianti M, Fourmosis I. Therapy of peri-implantitis: A systemic review. J Clin Periodontol. 2008;35(7):621-629.
14. Esposito M, Grusovin MG, Kakisis I, et al. Interventions for replacing missing teeth: treatment of peri-implantitis. Cochran Database Syst Rev. 2008;16(2):CD004970.
15. Renvert S, Polyzois I, Maguire R. Re-osseointegration on previously contaminated surfaces: a systemic review. Clin Oral Implants Res. 2009;20(Suppl 4):216-227.
16. Froum SJ, Froum SH, Rosen PS. Successful management of peri-implantitis with a regenerative approach: a consecutive series of 51 treated implants with 3- to 7.5-year follow-up. Int J Periodontics Restorative Dent. 2012;32(1):11-20.
17. Fransson C, Tomasi C, Pikner SS, et al. Severity and pattern of peri-implantitis-associated bone loss. J Clin Periodontal. 2010;37(5):442-448.
18. Serino G, Turri A. Outcome of surgical treatment of peri-implantitis: results from a 2-year prospective clinical study in humans. Clin Oral Implants Res. 2011;22(11):1214-1220.
19. Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25(4):229-235.
20. Froum SJ, Rosen PS. A proposed classification of peri-implantitis. Int J Periodontics Restorative Dent. 2012;32(5):533-540.
21. Park SH, Wang HL. Implant reversible complications: classification and treatments. Implant Dent. 2005;14(3):211-220.
22. Heitz-Mayfield LJA, Lang NP. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontol 2000. 2010;53:167-181.
23. Salvi GE, Aglietta M, Eick S, et al. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res. 2012;23(2):182-190.
24. Berglundh T, Zitzmann NU, Donati M. Are peri-implantitis lesions different from periodontitis lesions? J Clin Periodontol. 2011;38(Suppl 11):188-202.
25. Wilson TG. The positive relationship between excess cement and peri-implant disease: a prospective clinical endoscopic study. J Periodontol. 2009;80(9):1388-1392.
26. Wadhwani C, Pinyero A. Technique for controlling the cement for an implant crown. J Prosthet Dent. 2009;102(1):57-58.
27. Linkevicius T, Vindasiute E, Puisys A, Peciuliene V. The influence of margin location on the amount of undetected cement excess after delivery of cement-retained implant restorations. Clin Oral Impl Res. 2011;22(12):1379-1384.
28. Mombelli A, Nyman S, Bragger U, et al. Clinical and microbiological changes associated with an altered subgingival environment induced by periodontal pocket reduction. J Clin Periodontol. 1995;22(10):780-787.
29. Van Winkelhoff AJ, Goene RJ, Benschop C, Folmer T. Early colonization of dental implants by putative periodontal pathogens in partially edentulous patients. Clin Oral Implants Res. 2000;11(6):511-520.
30. Serino G, Ström C. Peri-implantitis in partially edentulous patients: association with inadequate plaque control. Clin Oral Implants Res. 2009;20(2):169-174.
31. Hultin M, Komiyama A, Klinge B. Supportive therapy and the longevity of dental implants: a systematic review of the literature. Clin Oral Implants Res. 2007;18(Suppl 3):50-62.
32. Roos-Jansåker AM, Lindahl C, Persson GR, Renvert S. Long-term stability of surgical bone regenerative procedures of peri-implantitis lesions in a prospective case-control study over 3 years. J Clin Periodontol. 2011;38(6):590-597.
33. Anner R, Grossmann Y, Anner Y, Levin L. Smoking, diabetes mellitus, periodontitis, and supportive periodontal treatment as factors associated with dental implant survival: a long-term retrospective evaluation of patients followed for up to 10 years. Implant Dent. 2010;19(1):57-64.
34. de Araújo Nobre M, Cintra N, Maló P. Peri-implant maintenance of immediate function implants: a pilot study comparing hyaluronic acid and chlorhexidine. Int J Dent Hyg. 2007;5(2):87-94.
35. Rapley JW, Swan RH, Hallmon WW, Mills MP. The surface characteristics produced by various oral hygiene instruments and materials on titanium implant abutments. Int J Oral Maxillofac Implants. 1990;5(1):47-52.
36. Louropoulou A, Slot DE, Van der Weijden FA. Titanium surface alterations following the use of different mechanical instruments: a systematic review. Clin Oral Implants Res. 2012;23(6):643-658.
About the Authors
Stuart J. Froum, DDS
Director of Clinical Research and Clinical Professor
Department of Periodontology and Implant Dentistry
New York University College of Dentistry
New York, New York

![Figure 4 A pre-surgical periapical radiograph of the mandibular right peri-implantitis-affected first molar implant. (Photo reproduced with permission from the American Academy of Periodontology.) (Reference: Froum SJ. Regenerative treatment for peri-implantitis affected implant: a case report. Clinical Advances in Periodontics. 2012. Figure 5 [accepted for publication])](/media/thumbnail/11070)
![Figure 5 A 7-year post-surgical periapical radiograph showing the bone fill of the defect. (Photo reproduced with permission from the American Academy of Periodontology.) (Reference: Froum SJ. Regenerative treatment for peri-implantitis affected implant: a case report. Clinical Advances in Periodontics. 2012. Figure 13. [accepted for publication])](/media/thumbnail/11071)