You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Patients ask for their dentists’ opinion about a variety of topics, with one of the most impactful being the possible treatment options for the replacement of a missing tooth or teeth. Without question, the discussion will and should include the alternative of dental implants to provide the patient with a fixed solution to their replacement needs. Clinicians can deliver extensive evidence-based detail on the success rates of dental implants with shockingly precise percentages. Any discussion of this modality of therapy should include a comparison—one that is based on the dental literature—of the success rates of dental implants to the long-term expectations associated with conventional fixed partial dentures (FPDs) as well as removable prosthetics and complete dentures.
The natural progression of this implant-related treatment planning process has led to the delivery of literally millions of dental implants over the past 30 years. The American Academy of Implant Dentistry has estimated that 5 million implants are now in place in the United States and an estimated 700,000 new implants are being placed each year—an enormous number by any measure.1 Historically placed in hospital and university settings by surgical specialists, dental implants are now being placed in private practices around the world by general dentists, prosthodontists, endodontists, and the surgical specialties of periodontics and oral surgery.
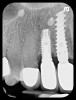
With all of the success seen in the dental community relative to implant placement, dentists are now seeing a more pronounced trend in dental implant care: an increase in the bacteriologic and/or traumatogenic occlusion-mediated loss of tissue integrity, accompanied many times by alveolar bone loss. The most common terminology applied to this condition is peri-implantitis (Figure 1). As replete as the dental literature is with success story after success story, there is virtually no detailed and specific prospective clinical trial data on the diagnosis and management of the peri-implant diseases, which result in soft tissue inflammation and the potential for alveolar bone destruction that may lead to the loss of the implant.
In other words, dentists have no guidelines available in their decision-making armamentarium to direct the development of a treatment plan when observing the onset or results of peri-implant disease. In fact, it was not until very recently that appropriate terms were defined to describe the disease process and to provide the clinician with the distinction between the inflammatory tissue component alone and a combination of soft and hard tissue disease states. Therefore, no protocol or recipe for management has been established.
An important question at the heart of this discussion is, “Why is the dental community now seeing so many articles devoted to peri-implant disease?” Is the incidence of peri-implant disease on the rise? Is the overall success rate of dental implants declining? How does this relate to a multitude of potentially contributing causes such as: (1) changes in the surface of the implant; (2) increases in the number of medically compromised patients having implants placed; (3) increases in the number of smoking patients having implants placed; (4) increases in nonsurgical specialists and general dentists placing implants; (5) better recognition of peri-implant disease; (6) a greater number of patients with previously placed implants now being followed in hygiene programs; or (7) simply increases in the number of implants being placed today? The obvious point to this discussion is to determine acceptable and predictable treatment modalities for the management of peri-implant disease.
As a preface to this discussion, it should be noted that a key component to the prevalence of peri-implant disease is to do everything possible to prevent it in the first place. This includes a purposeful discussion with the patient about long-term expectations and the possible need for further care to treat a potential peri-implant disease situation. Secondly, with respect to the smoking patient and/or a medically compromised case, a better understanding of the impact of placing an implant into a compromised environment may lead to a more meaningful discussion in terms of other options besides a dental implant perhaps being more appropriate for those particular scenarios.
Likewise, a clinician who is less skilled at the proper management of peri-implant soft and hard tissues as well as less skilled in the placement of the dental implant in the correct anatomical location and at the most ideal angulation may result in an indirect negative implication regarding the long-term susceptibility of a dental implant to peri-implant disease.2 Finally, substantial increases in the number of dental implants being placed will statistically lead to an increase in the number of implants that experience an adverse event, such as fracture of the implant body, fracture of the retention screw, fracture of the porcelain, and the incidence of the peri-implant disease process.
Prevention
The most reasonable way to avoid a peri-implant disease situation is to be proactive and preventive in the decision-making process regarding options for tooth replacement. It is vital to understand the relationship of smoking to peri-implant diseases and to be able to relay to the patient the risks involved relative not only to failure rates, but also potential problems over the lifespan of the implant.3 It has been shown that patients who smoke exhibit a higher rate of failure, a higher rate of peri-implantitis, and a decreased response to therapy when treating peri-implant disease.4
Patients who have a history of periodontal disease and are having dental implants placed should be counseled on the need to maintain a more frequent supportive periodontal therapy regimen, which, ideally, would include an alternating schedule with a periodontist. Long-term tooth retention and a decrease in the recurrence of periodontitis/peri-implantitis have been shown in the literature to improve when patients adhere to a prescribed protocol.5,6 In addition, giving the patient specific home-care instructions—especially if they have a history of inefficient home care that has resulted in an increased caries rate and/or susceptibility to periodontal disease—must be part of the discussion. It is always important to consider why the patient lost his or her teeth in the first place.
Finally, a clear understanding of the patient’s systemic condition must be an integral part of the decision-making process. For example, dentists are now more acutely aware of the role that diabetes plays in wound healing and, most recently, the impact bisphosphonate medications have on the potential for compromised healing in extraction sockets7 and the potential issues that are associated with the integration process of dental implants.8
Diagnosis
Recognition of the initiation and progressive nature of a disease state is vital to making a decision about the appropriate treatment modality. In addition, the question must be posed as to whether or not the same treatment protocol will apply to a dental implant in the maxillary anterior segment as it would to an implant in the posterior aspect of the mouth. Compromise of the health of the surrounding gingival tissue and/or the supporting alveolar bony complex of a dental implant also should have meaningful terminology to relay an appropriate diagnosis to the patient, the insurance companies, and to the supporting dental team (which is typically the surgeon and their restorative colleagues).
Recently, Rosen and Froum9 defined peri-implant mucositis as inflammation of the gingival complex surrounding a dental implant that results from a bacterial infection without involvement of the underlying bony support. This was further clarified with the inclusion criteria for the presence or absence of keratinized gingiva relative to the terms peri-implant mucositis (with no keratinized gingiva) versus peri-implant gingivitis (with keratinized gingiva), as it is apparent that keratinized gingiva is important to the long-term success of dental implants. In addition, peri-implantitis was defined as inflammation of the gingival complex surrounding a functionally loaded dental implant with loss of supporting alveolar bone that results from a bacterial infection.10
It is interesting to note that the definition of peri-implantitis includes the term functional load. Dental implants can certainly exhibit gingival inflammation and alveolar bone loss before the restoration is fabricated, be it the provisional or final prosthesis. With that said, the term peri-implantitis should apply to all implants with radiographic and clinical bone loss exceeding that which is historically defined as being within normal limits. This may occur during the osseointegration phase of therapy or after fabrication of a functional restoration.
Implant success criteria had been previously defined by the NIH-Harvard Conference in 1978 and was established as not more that 2 mm of bone loss in the first year of loading and less than 0.2 mm per year thereafter.11 Data is needed to determine at what point abnormal or excess bone loss has occurred and can be considered a disease process. In 2012, Froum and Rosen12 proposed a peri-implantitis disease classification system based on evidence of bleeding and/or suppuration, probing depth, and percentage of radiographic bone loss. This classification system is defined as early, moderate, and severe, and it provides the clinician with a distinct set of criteria-based parameters to use to define a specific diagnosis.
Treatment Options
Management of peri-implant diseases, for the most part, is analogous to treatment options for conventional periodontal diseases. The dental literature offers the following options for care of soft and/or hard tissue inflammatory destruction around a dental implant. (Please note this article does not attempt to discuss every available option, but it does provide a concise summary.)
Scaling and Root Planing
In the event that the treating clinician has determined that the peri-implant disease has been limited to the surrounding mucosal tissue (mucositis), then a closed approach involving scaling and root planing (SRP) may be undertaken.13 The etiology in this situation is very often cement extrusion under a cement-retained restoration. Linkevicius et al demonstrated peri-implant disease in 85% of cement remnant cases.14 Alternatively, only 1.08% of cases demonstrated peri-implantitis in screw-retained restorations.
Systemic/Local Antibiotics
The addition of a systemic or local-delivery pharmacotherapeutic agent to the treatment protocol involving a closed approach with SRP has been shown to be of limited value.15 The problem with managing the peri-implant microbiota with antibiotic therapy is the growing trend of bacteria demonstrating increased resistance to multiple, commonly used medications. Rams et al evaluated 160 dental implants with peri-implantitis in 120 adult patients.16 There was an increased rate of resistance to clindamycin, amoxicillin, doxycycline, and metronidazole when used alone, ranging from 21.7% to 46.7%. However, when amoxicillin and metronidazole were combined, the resistance rate dropped to 6.7%.
Open Flap Debridement
An open flap debridement (OFD) approach with implant surface detoxification and/or implantoplasty17 is defined as the removal of the threads or surface structure of the implant in a mechanical fashion (ie, a finishing or polishing bur, rotary titanium or stainless steel brushes, or hand instrumentation). The clinician should be cautious when using high-speed finishing burs. This process can significantly weaken the implant by thinning the titanium walls. Chan et al18 evaluated 32 tapered implants; half were 4.7 mm and half were 3.75 mm. Implantoplasty was completed and an off-axis load at 30° was applied. All of the 3.75-mm implants fractured. Relative to groove/thread removal, it has also been shown that smoothing the roughened surface did not significantly improve the clinical outcome.
Surgical Therapy
A surgical approach in which OFD in combination with surface detoxification, placement of a bone graft, and the use of a systemic antibiotic would appear to have its advantages. However, there have been several reports from both sides of the fence. In one study by Renvert et al,19 surgical therapy was shown to arrest peri-implant bone loss over 5 years by decreasing suppuration, bleeding on probing, and probing depths, whereas Esposito et al20 showed that simple mechanical procedures were equivalent to complex procedures. The results relative to the decision to use an adjunctive systemic antibiotic with any of these treatment options remains unclear and debatable.
Guided Bone Regeneration
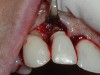
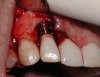
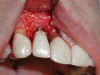
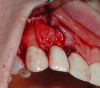
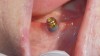
A guided bone regeneration (GBR) approach involving implant surface detoxification (Figure 2), placement of a bone graft combined with a barrier membrane (Figure 3 and Figure 4), and use of a systemic antibiotic has demonstrated the most predictable improvement in peri-implant parameters after destruction of supporting bone. GBR procedures demonstrated reductions in probing depth of 3.16 mm and 2.1 mm of radiographic bone fill in a recent systematic review and meta-analysis of 21 studies by Chan et al.21 Froum and Rosen22 defined a specific protocol based on reentry evaluation of previously treated peri-implantitis sites that included the use of rhPDGF-BB with freeze-dried mineralized bone and the addition of a resorbable membrane. Nonresorbable Gore-Tex® sutures were used to approximate the gingival tissue and were retained in place for 2 weeks. The patient was also kept on chlorhexidine for 2 weeks postoperatively. The reentry photographs from this report are quite impressive. However, the authors do caution the interpretation of re-osseointegration (Figure 5 and Figure 6).
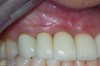
Finally, whether managing adult periodontitis or peri-implantitis (Figure 7), surgical design and soft tissue manipulation should be considered of vital importance, particularly in the maxillary anterior esthetic zone. Careful attention to papilla preservation techniques23 and incision design to minimize or eliminate the potential for gingival recession are vital to maintaining a natural-looking gingival framework around the implant restoration. This includes the need for adequate keratinized tissue because, even in the most precise cone-beam computed tomography-guided implant placement procedures, studies have shown that the absence of a residual band of keratinized tissue >6 mm wide may be associated with an increase in clinical attachment loss (Figure 8) and an increase in dehiscences after 1 year.24
Implant Surface Detoxification
A seemingly essential component to the treatment of peri-implantitis is detoxification of the implant surface. With so many proprietary surfaces encompassing the current implant market, it is difficult determine the most appropriate way to eliminate the bacteriologic pathogens that contribute to bone loss. Historical measures have included the use of implantoplasty to remove the threads/grooves of the implant as well as the use of antibiotics and chemical agents. Following are some of the current considerations for detoxification of the implant surface.
Mechanical/Physical Detoxification
The use of a finishing bur on a high-speed handpiece in combination with scalers to eliminate the threads or the contaminated surface structure of an implant was described by Lang et al25 in the treatment of peri-implantitis with specific regard to hydroxyapatite-coated implants. This implantoplasty technique certainly has its challenges and concerns as previously mentioned.
Photodynamic Therapy
Photodynamic therapy involves the use of a low-power laser in combination with an appropriate photosensitizer to increase detoxification of the implant surface.26
Laser Detoxification
This has been a widely studied treatment option for the obliteration and removal of bacterial contaminants. Laser options that have been evaluated include CO2, diode, Er:YAG, Nd:YAG, and Ho:YAG. Interestingly, the Nd:YAG and Ho:YAG lasers have been shown to have varying effects on the surface of the implant relative to not only decontamination, but also to damaging the surface and creating a surface that is not conducive to repair.27 Mellado-Valero et al28 showed that the CO2 and diode lasers demonstrated the best results with decontamination, no alteration of the implant surface, and without a significant increase in temperature of the implant body.
Chemical/Antibiotic Decontamination
Multiple agents have been used to treat the surface of a diseased dental implant. These include but are not limited to chlorhexidine, hydrogen peroxide, tetracyclines, citric acid, EDTA, phosphoric-acid gel, and sterile saline. Citric acid and tetracycline have demonstrated some efficacy but, overall, no chemical agent has demonstrated clear effectiveness as a monotherapy for the treatment of peri-implantitis.29
Maintenance/Supportive Periodontal Therapy
As with any recipe, each ingredient is important to the final product. This is certainly true regarding the component of dental implant care associated with maintenance of the long-term health of the remaining dentition as well as the implants replacing the natural teeth. The importance of supportive periodontal care cannot be overstated, as Esposito et al20 reported that in patients who originally presented with severe bone loss and surgical intervention to repair/stabilize progressive peri-implantitis, the peri-implantitis returned 100% of the time. Therefore, recognition and early intervention should be the overall goal. Rocuzzo et al30 found that there was a statistically significant higher number of sites that required additional surgery or antibiotic therapy when patients did not adhere to a supportive periodontal therapy regimen.
There are certainly some staggering numbers in the clinical arena relative to the incidence of peri-implant mucositis and peri-implantitis. Fardal and Grytten31 found that the prevalence of peri-implant disease was 31.1%. When comparing natural teeth to dental implants in this maintenance patient population, it was reported that the mean number of disease-free years with a dental implant was 8.66, while the neighboring teeth exhibited 9.08 years and the contralateral teeth 9.93 years. Most interestingly, the cost of maintaining dental implants over time was five times greater than the cost associated with maintaining natural teeth. Likewise, Atieh et al,32 in a systematic review and meta-analysis of 1,497 patients with 6,283 implants, estimated the rate of peri-implant mucositis at 30.7% and peri-implantitis at 9.6%. There was a significant impact in smoking patients, showing a two-times greater incidence. Again, when patients were compliant with a supportive periodontal maintenance hygiene program, the incidence of peri-implant disease was significantly decreased.
Conclusion
It is apparent that peri-implant disease is a challenging problem for all dental practitioners. It is incumbent upon the general dentist, nonsurgical specialist, and surgical specialist to have open and honest conversations with their patients regarding the prevalence and potential complications of peri-implantitis before placing a dental implant. The dental community can no longer lead patients to believe that dental implants are beyond disease problems. It is important from an informed consent and patient education perspective that these discussions occur.
With that said, dental implants are still, and deservedly should be, recognized as having the greatest success rates of any tooth-replacement option (Figure 9). To be on the highest end of the success rate, patients with systemic confounding factors, those who smoke, and those with a history of periodontal disease33 need to be counseled on appropriate home care instruction, the need to enroll in a smoking cessation program, maintenance of a lifestyle that is consistent with promoting systemic health, and the need to be compliant with a long-term supportive periodontal maintenance program. Patients must take an active role in their care so that the recipe for success will be complete.
References
1. Millenium Research Group. US market for dental implants 2003. Global Dental Series. Toronto, Ontario: January 2003.
2. Da Silva JD, Kazimiroff J, Papas A, et al. Outcomes of implants and restorations placed in general dental practices: A retrospective study by the Practitioners Engaged in Applied Research and Learning (PEARL) network. J Am Dent Assoc. 2014;145:704-713.
3. Clementi M, Rossetti PH, Penarrocha D, et al. Systemic factors for peri-implant bone loss: A systemic review and meta-analysis. Int J Oral Maxillofac Surg. 2014;43:323-334.
4. Rodriguez-Argueta OF, Figueiredo R, Valmageda-Castellon E, Gay-Escoda C. Postoperative complications in smoking patients treated with implants: a retrospective study. J Oral Maxillofac Surg. 2011;69:2152-2157.
5. Axelsson P, Lindhe J. The significance of maintenance care in the treatment of periodontal disease. J Clin Periodontol. 1981;8:281-294.
6. Costa FO, Takenaka-Martiniez S, Cota LO, et al. Peri-implant disease in subjects with and without preventative maintenance: a 5-year follow up. J Clin Periodontol. 2012;39:173-181.
7. Kim I, Ki H, Lee W, et al. The effect of systemically administered bisphosphonates on bony healing after tooth extraction and osseointegration of dental implants in the rabbit maxilla. Int J Oral Maxillofac Implants. 2013;28:1194-1200.
8. Yip JK, Burrell LN, Cho SC, et al. Association between oral bisphosphonate use and dental implant failure among middle-aged women. J Clin Periodontol. 2012;39:408-414.
9. Rosen PS, Froum SJ. The need for consensus. Int J Periodontics Restorative Dent. 2014;34:7-8.
10. Lindhe J, Meyle J. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35(8 Suppl):282-285.
11. Dental Implants: Benefit and Risk. NIH Consensus Statement. 1978;Jun 13-14;1(3):13-19.
12. Froum SJ, Rosen PS. A proposed classification for peri-implantitis. Int J Periodontics Restorative Dent. 2012;32:533-540.
13. Renvert S, Roos-Jansaker AM, Claffey N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol. 2008;35(8 Suppl):305-315.
14. Linkevicius T, Puisys A, Vindasiute E, et al. Does residual cement around implant-supported restorations cause peri-implant disease? A retrospective case analysis. Clin Oral Implants Res. 2012 Aug 8. doi: 10.1111/j.1600-0501.2012.02570.x. [Epub ahead of print]
15. Javed F, Al Ghamdi AS, Ahmed A, et al. Clinical efficacy of antibiotics in the treatment of peri-implantitis. Int Dent J. 2013;63:169-176.
16. Rams TE, Degener JE, van Winklehoff AJ. Antibiotic resistance in human peri-implant microbiota. Clin Oral Impl Res. 2014;25:82-90.
17. Suh JJ, Simon Z, Jeon YS, et al. The use of implantoplasty and guided bone regeneration in the treatment of peri-implantitis: two case reports. Implant Dent. 2003;12:277-282.
18. Chan HL, Oh WS, Ong HS, et al. Impact of implantoplasty on strength of the implant-abutment complex. Int J Oral Maxillofac Implants. 2013;28:1530-1535.
19. Renvert S, Polyzois I, Claffey N. Surgical therapy for the control of peri-implantitis. Clin Oral Implants Res. 2012;23(Suppl 6):84-94.
20. Esposito M, Grusovin MG, Worthington HV. Interventions for replacing missing teeth: treatment of peri-implantitis. Cochrane Database Syst Rev. 2012 Jan 18;1:CD004970. doi: 10.1002/14651858.CD004970.pub5.
21. Chan HL, Lin GH, Suarez F, et al. Surgical management of peri-implantitis: a systematic review and meta-analysis of treatment outcomes. J Periodontol. 2013 Nov 21. [Epub ahead of print]
22. Froum SJ, Rosen PS. Reentry evaluation following treatment of peri-implantitis with a regenerative approach. Int J Periodontics Restorative Dent. 2014;34:47-59.
23. Takei HH, Han TJ, Carranza FA Jr, et al. Flap techniques for periodontal bone implants: papilla preservation techniques. J Periodontol. 1985;56:204-210.
24. Malo P, Rigolizzo M, Nobre MD, et al. Clinical outcomes in the presence and absence of keratinized mucosa in mandibular guided implant surgeries: a pilot study with a proposal for the modification of the technique. Quintessence Int. 2013;44:149-157.
25. Lang NP, Wilson TG, Corbet EF. Biological complications with dental implants: their prevention, diagnosis and treatment. Clin Oral Implants Res. 2000;11(Suppl 1):146-155.
26. Bombeccari GP, Guzzi G, Gualini F, et al. Photodynamic therapy to treat peri-implantitis. Implant Dent. 2013;22:631-638.
27. Kreisler M, Götz H, Duschner H. Effect of Nd:YAG, Ho:YAG, Er:YAG, CO2 , and GaAIAs irradiation on surface properties of endosseous dental implants. Int J Oral Maxillofac Implants. 2002;17:202-211.
28. Mellado-Valero A, Buitrago-Vera P, Solá-Ruiz MF, Ferrer-Garcia JC. Decontamination of dental implant surface in peri-implantitis treatment: a literature review. Med Oral Patol Oral Cir Bucal. 2013;18:e869-e876.
29. Claffey N, Clarke E, Polyzois I, Renvert S. Surgical treatment of peri-implantitis. J Clin Periodontol. 2008;35:316-332.
30. Roccuzzo M, Bonino L, Dalmasso P, Aglietta M. Long-term results of a three arms prospective cohort study on implants in periodontally compromised patients: 10-year data around sandblasted and acid-etched (SLA) surface. Clin Oral Implants Res. 2013 Jul 19. doi: 10.1111/clr.12227. [Epub ahead of print]
31. Fardal O, Grytten J. A comparison of teeth and implants during maintenance therapy in terms of the number of disease-free years and costs—an in vivo internal control study. J Clin Periodontol. 2013;40:645-651.
32. Atieh MA, Alsabeeba NH, Faggion CM Jr, Duncan WJ. The frequency of peri-implant diseases: a systematic review and meta-analysis. J Periodontol. 2013;84:1586-1598.
33. Renvert S, Persson GR. Periodontitis as a potential risk factor for peri-implantitis. J Clin Periodontol. 2009;36(Suppl 10):9-14.
About the Author
Christopher R. Richardson, DMD, MS
Private Practice
Richmond, Virginia
Clinical Faculty
Virginia Commonwealth University School of Dentistry
Richmond, Virginia